Our Enabling
Technology
Bio-Courier® is a hybrid organic–inorganic delivery platform that combines lipids and bioabsorbable silicon for improved stability and enhanced RNA delivery properties.*
Unlike conventional lipid nanoparticles, Bio-Courier technology separates initial manufacture from RNA loading, removing the need for cold chain distribution and promoting global accessibility.
The Bio-Courier platform is readily customisable for a wide range of applications, including tissue-specific targeting and multiple administration routes.
The technology achieves robust transfection efficiency, even with hard-to-transfect primary cells and with in vivo targets that are beyond the reach of current lipid nanoparticle formulations.
The Bio-Courier platform offers extensive freedom to operate. The combination of biodegradable silicon with lipids makes it possible to design unique lipid nanoparticles not covered by well-known patent rights.
Bio-Courier is a registered trademark in the UK, EU, China, Japan, South Korea and Australia. Trademark applications in Canada and USA are pending.
RNA Delivery
The Fundamental Challenge
Nucleic acid therapeutics have tremendous potential to transform the treatment of a wide range of conditions. However, this new generation of medicines relies critically on suitable delivery systems because ‘naked’ RNA is rapidly metabolised and cleared after administration. Without an appropriate carrier, it can also provoke unwanted inflammatory and immune responses.
Effective delivery technologies are therefore essential to protect therapeutic RNA against degradation, to direct it to the desired target tissues and cells, and to avoid adverse effects. Once taken up by cells, these delivery systems must also ensure efficient release of the RNA payload into the cytoplasm.
Lipid nanoparticles (LNPs) are the current mainstay and represent the most widely used delivery mechanism for RNA therapeutics in the clinic. However, they have a number of important drawbacks that limit wider application. The key challenges for current LNPs are manufacturing, stability, safety, tissue-specific targeting and transfection efficiency.
SiSaf’s proprietary Bio-Courier technology overcomes these challenges.
Bio-Courier®Technology
The Solution
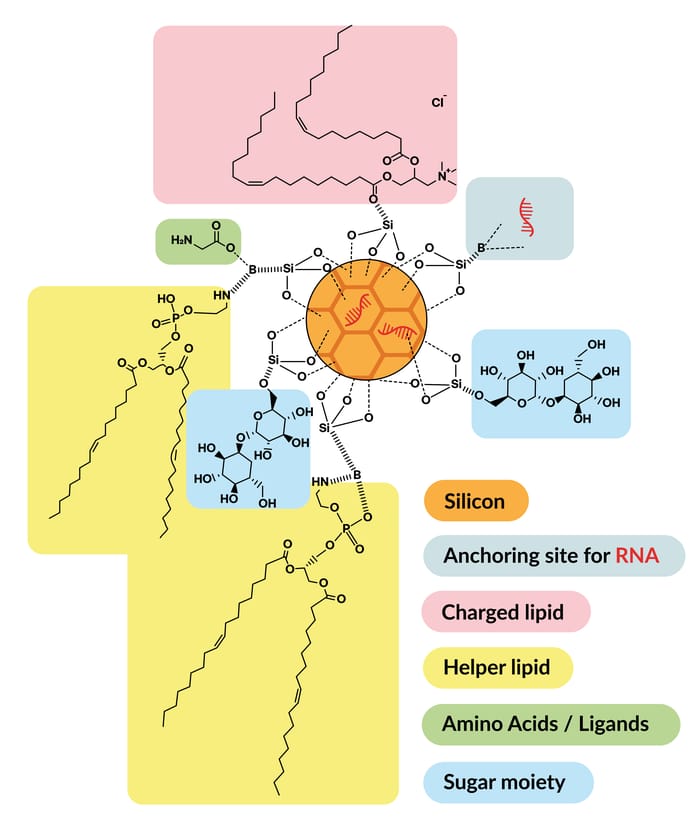
SiSaf’s Bio-Courier technology addresses the challenges of lipid nanoparticles by stabilizing both lipids and RNA through a bioabsorbable silicon matrix.
Silicon’s positive charge and high ζ-potential electrostatically bind and condense RNA preventing the premature disassociation of RNA and reducing the reliance on cationic or ionisable lipids.
High volumes of tightly condensed RNA are electrostatically bonded to the silicon matrix, protecting them from hydrolysis to prolong their systemic survival.
The structural integrity of the silicon metalloid prevents physical collapse of the particle permitting lyophilization whilst retaining RNA integrity.
Tailored surface lipids combined with pH dependant silicon/RNA bond dissolution maximize transfection efficiency.
The versatility of particle size, surface charge and the addition of surface ligands enable passive or active cell targeting and multiple administration routes.

SiSaf’s Bio-Courier technology addresses the challenges of lipid nanoparticles by stabilizing both lipids and RNA through a bioabsorbable silicon matrix.
Silicon’s positive charge and high ζ-potential electrostatically bind and condense RNA preventing the premature disassociation of RNA and reducing the reliance on cationic or ionisable lipids.
High volumes of tightly condensed RNA are electrostatically bonded to the silicon matrix, protecting them from hydrolysis to prolong their systemic survival.
The structural integrity of the silicon metalloid prevents physical collapse of the particle permitting lyophilization whilst retaining RNA integrity.
Tailored surface lipids combined with pH dependant silicon/RNA bond dissolution maximize transfection efficiency.
The versatility of particle size, surface charge and the addition of surface ligands enable passive or active cell targeting and multiple administration routes.
Manufacturing
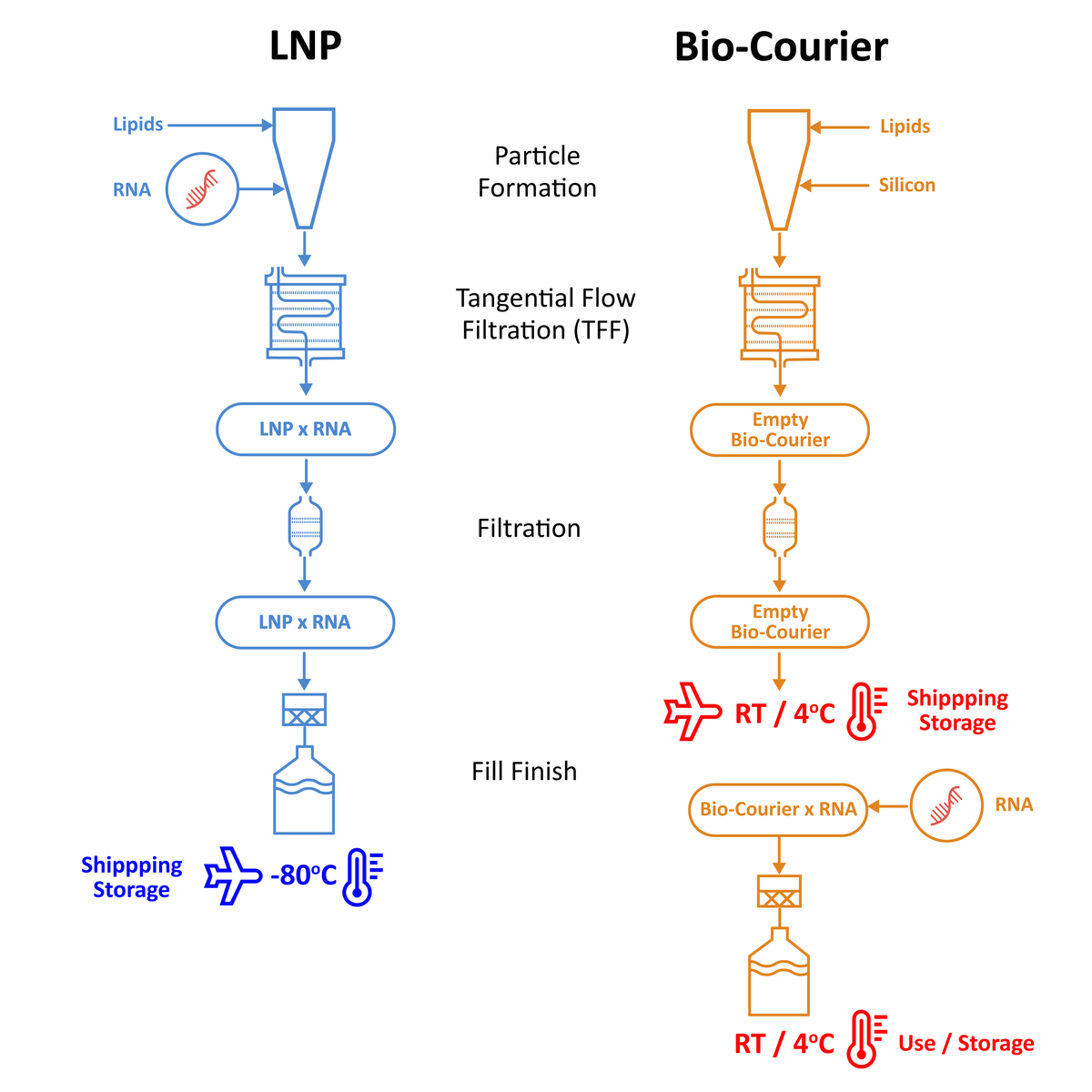
Unlike conventional LNPs, Bio-Courier can be manufactured ‘empty’ and quickly loaded with any desired RNA therapeutic at the point of use. This eliminates the need for ultracold chain distribution and overcomes other issues due to RNA instability.
By separating carrier manufacture from API loading, Bio-Courier technology can extend global accessibility to regions where end-to-end ultracold chain distribution is not feasible and it can reduce the environmental impact associated with ultracold transport and storage.
The modular nature of Bio-Courier also opens up new possibilities in rare disease treatments and personalized RNA medicine, as it permits the on-demand preparation of customized RNA-loaded nanoparticles on any scale at the point of use.
Stability

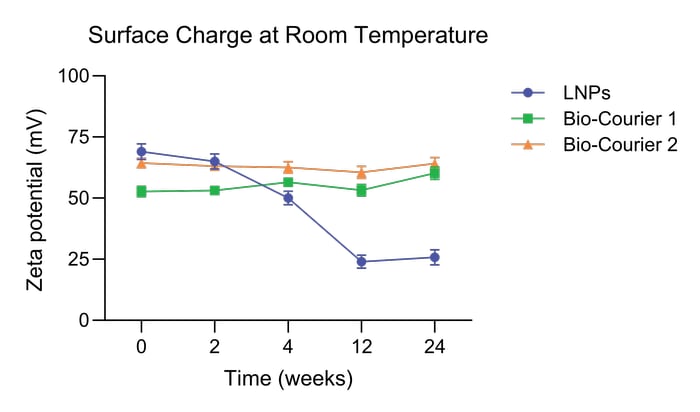
Bio-Courier was developed with increased stability and shelf life in mind. The silicon component significantly enhances physical and chemical stability, enabling particles to retain their original properties for extended periods of time, even at room temperature.
In long-term storage tests, Bio-Courier silicon stabilized lipid nanoparticles maintained their original size and zeta potential for at least 80 weeks at 4 °C and 24 weeks at room temperature.
Bio-Courier formulations are also more resistant to shear stress. Their polydispersity index (PDI) is unaffected by multiple cycles of extrusion through a 30 nm membrane, unlike LNPs formulated without silicon.


Safety
PEGylated lipids are an essential structural and functional component of LNPs but they can elicit unwanted immune responses, compromising both safety and efficacy.
In Bio-Courier, the stabilizing effect of the silicon component removes the need for PEGylation without introducing any additional safety issues.
The hydrolysable silicon component of Bio-Courier particles is designed to degrade in vivo to 100% biocompatible orthosilicic acid, which is bioabsorbable and has no known safety concerns.
Targeting

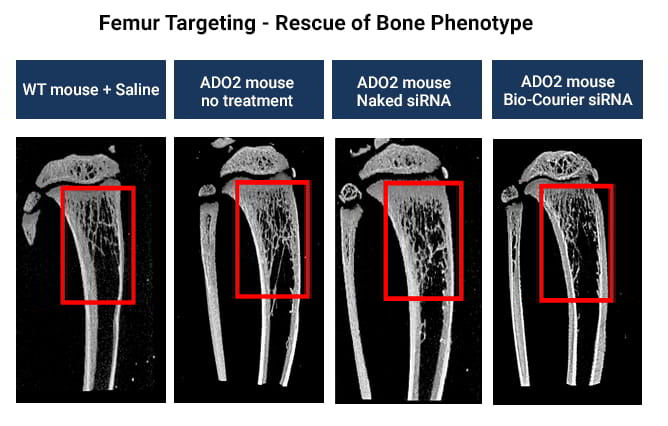
Targeting of specific tissues or cell types is extremely challenging with standard LNPs due to their strong tendency to accumulate in the liver. In contrast, Bio-Courier formulations can readily be fine-tuned for RNA delivery to the desired site of action.
In a mouse model of Osteopetrosis type 2 (ADO2), Bio-Courier achieved optimal delivery of siRNA targeting the mutant Clcn7 allele to femur, resulting in the rescue of the bone phenotyope.
Transfection Efficiency
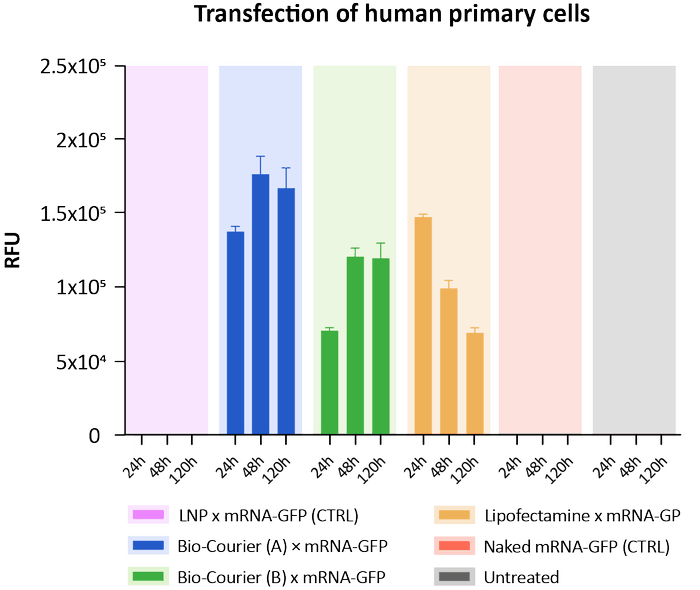
Low transfection efficiency is one of the bottlenecks with current LNP formulations, due to their limited capacity to escape from endosomes following cellular uptake. Bio-Courier’s unique properties enable greatly improved transfection performance, even in hard-to-transfect primary cells and in vivo models.
In a transfection experiment with human primary cells, Bio-Courier sshLNP formulations showed similar mRNA transfection efficiency to a commercial reagent (positive control), while LNPs with the same lipid composition as the sshLNPs but without silicon hybridisation were completely ineffective at the concentrations used. This highlights the critical role of the bioabsorbable silicon matrix in sshLNPs.
In luciferase reporter mice, siRNA-loaded sshLNPs achieved successful gene knockdown with topical ocular administration.

Publications
Bio-Courier: a new delivery technology to increase the global accessibility of RNA therapeutics
Suzanne Saffie-Siebert, Andy Gibson, Ashkan Dehsorkhi
October 2023
Manufacturing Chemist (online)
Novel hybrid silicon-lipid nanoparticles deliver a siRNA to cure autosomal dominant osteopetrosis in mice. Implications for gene therapy in humans
September 2023
Molecular Therapy – Nucleic Acids, VOLUME 33, P925-937
Delivery of siRNA to the Eye
2021
In: Ditzel, H.J., Tuttolomondo, M., Kauppinen, S. (eds) Design and Delivery of SiRNA Therapeutics. Methods in Molecular Biology, vol 2282. Humana, New York, NY, pp 443–453
Topical siRNA delivery to the cornea and anterior eye by hybrid silicon-lipid nanoparticles
October 2020
Journal of Controlled Release, Volume 326, 2020, pp 192-202
Effective Small Interfering RNA Therapy to Treat CLCN7-dependent Autosomal Dominant Osteopetrosis Type 2
January 2015
Molecular Therapy – Nucleic Acids (2015) 4, e248
Nanotechnology approaches to solving the problems of poorly water-soluble drugs
August 2005
Drug Discovery World, Summer 2005
High Yield Incorporation of Plasmid DNA within Liposomes: Effect on DNA Integrity and Transfection Efficiency
Gregory Gregoriadis, Roghieh Saffie & Stephen Hart
1996
Journal of Drug Targeting, 3:6, 469-475





