Pipeline
SiSaf is developing a growing pipeline of RNA therapeutics, with a focus on oncology. Our lead program is a microRNA treatment of solid tumors, with the first indication being pancreatic cancer.
Bio-Courier technology has also shown great promise in delivering APIs to bone and chondrocytes and we are developing siRNA treatments for rare skeletal disorders, Osteopetrosis ADO2 and Achondroplasia.
These therapeutics utilize the fundamental features of the company’s proprietary Bio-Courier delivery platform: durable RNA stabilization, non-immunogenic, efficient targeting and transfection, and no accumulation.
SiSaf’s Bio-Courier technology has also been licensed to partners, illustrating the impact the technology can have in delivering therapeutic benefits to patients. A topical Bio-Courier formulation for the treatment of Autoimmune Alopecia has successfully completed a clincial Phase 2 trial and is moving to Phase 3. We have also partnered with a leader in ophthalmic genetic disease to develop the world’s first eye drop RNA interference therapy and CRISPR cas 9 gene editing cure for Corneal Dystrophy.

In-House Programs
Partnered Programs
In-House
Pancreatic Cancer
SIS-401-PACA is a novel miRNA-based treatment approach for solid tumors, with pancreatic ductal adenocarcinoma being the first indication.
According to the American Society of Clinical Oncology (“ASCO”), in 2020, an estimated 496,000 people were diagnosed with pancreatic cancer globally of which an estimated 94% died from the disease. The 5-year survival rate for people with pancreatic cancer in the U.S. is 11%.
SIS-401-PACA uses miRNA replacement to induce a much more complete eradication of the tumor, reduce the spread of cancer to other organs and increase the long-term survival in patients. It can be combined with chemotherapy and data indicate that it will also be efficacious in chemoresistant cells.

In-House
Metastatic Cancer
SIS-402-MST is a siRNA therapy designed to inhibit a protein that plays a critical role in tumor progression and metastasis, with Bio-Courier minimizing off target effects.
Even though modern medicine is able to treat many primary tumors successfully, cancers often spread to other organs. Every third breast or lung cancer patient develops metastatic disease. SIS-402-MST aims to prevent metastasis in early-stage cancers and avert residual cancer progression after chemotherapy or cancer dormancy and reactivation during remission.

In-House
Hypertrophic & Keloid Scars
Non Melanoma Skin Cancer
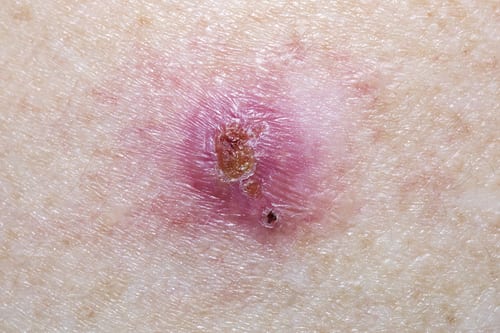
SIS-022 is a reformulation of 5-fluorouracil (5-FU) for the topical treatment of hypertrophic and keloid scars, and for the non-surgical treatment of precancerous lesions (actinic keratosis) and non melanoma skin cancer (Bowen’s Disease and basal cell carcinoma).
No effective non-invasive treatment of hypertrophic and keloid scars is available and SIS-022 will offer the first efficacious topical treatment option. It will also offer an alternative to existing topical treatments of actinic keratosis and non melanoma skin cancer with fewer side effects.

In-House
Autosomal Dominant Osteopetrosis (ADO2)
SIS-101-ADO is a systemic Bio-Courier siRNA designed to inhibit the dominant-negative mutations of the Clcn7 gene that cause Autosomal Dominant Osteopetrosis (ADO2), in order to rescue the bone phenotype.
ADO2 is a genetic bone disease affecting around one in 20,000 people. The mutated gene causes a defect in bone reabsorption by osteoclasts resulting in increased bone density. This can lead to vision loss, osetomyelitis, or bone marrow failure and most patients suffer from a high rate of bone fractures.
Currently, there is no cure for ADO2 and SIS-101-ADO represents a first-in-class treatment to reverse the bone phenotype to normal.
The U.S. FDA has granted SOS-101-ADO Orphan Drug Designation. In addition, due to the serious manifestations of this rare skeletal disorder in children, SIS-101-ADO has been granted Rare Pediatric Disease Designation for the treatment of Autosomal Dominant Osteopetrosis. This entitles SiSaf to apply for a priority review voucher (RPD PRV).
In-House
Achondroplasia

SIS-102-ACH is a systemic Bio-Courier siRNA designed to inhibit mutated Fibroblast Growth Factor Receptor 3 gene (FGFR3), a negative regulator of bone growth, and rescue the phenotype of patients with Achondroplasia.
Around one in 25,000 people are born with Achondroplasia, a disorder of bone growth that prevents the changing of cartilage (particularly in the long bones of the arms and legs) to bone. It is characterized by dwarfism, limited range of motion at the elbows, large head size (macrocephaly), and small fingers. Achondroplasia can cause health complications such as interruption of breathing (apnea), obesity, recurrent ear infections, and an exaggerated inward curve of the lumbar spine (lordosis). More serious problems include a narrowing of the spinal canal that can pinch (compress) the upper part of the spinal cord (spinal stenosis) and lead to a build-up of fluid in the brain.
SIS-102-ACH will be the first RNA therapeutic that addresses the root cause of Achondroplasia, directly inhibiting the mutated FGFR3 gene without affecting its healthy copy. Unlike other therapies, SIS-102-ACH encourages the growth of all bones in the body such as arms, hands and skull, and not just in the legs. It can be dosed by injection weekly or at longer intervals. While our initial focus is on pediatric patients, we are hopeful that in time we can also demonstrate therapeutic benefits for adults.

Partnered
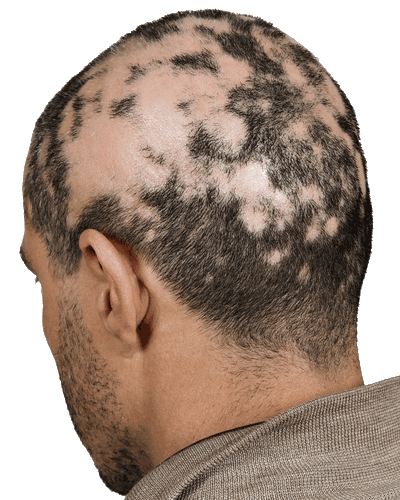
Alopecia Areata
SIS-302-AA is treating Alopecia Areata (AA). This is one of the most common and psychologically distressing human autoimmune conditions. In the U.S. alone, over 6 million individuals are estimated to be affected by Alopecia Areata at some point during their lifetime. It is distinguished by non-scarring patchy hair loss on the scalp and, in some individuals, pitting on the nails.
SIS-302-AA is being developed by a UK partner and is a reformulation of an approved drug molecule with Bio-Courier. It is aimed at treating mild to moderate Alopecia. The formulation with Bio-Courier is designed to stabilize the API and enhance its penetration into skin and hair follicles. SIS-302-AA has successfully completed a Phase 2 trial, meeting the primary endpoint with no major adverse events, and is now moving to Phase 3.
Partnered
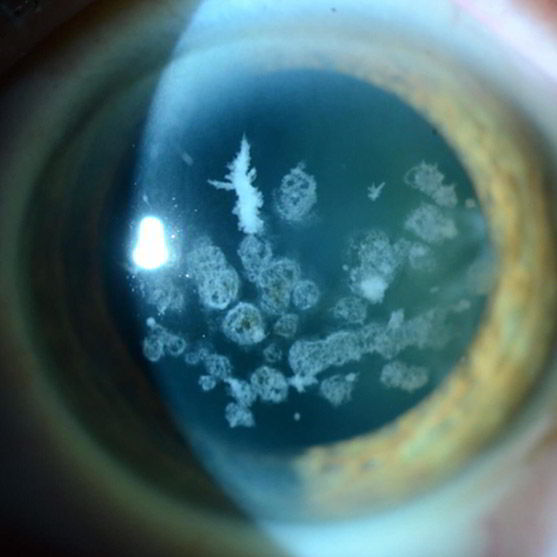
Type II Corneal Dystrophy

SIS-201-CD is a topical Bio-Courier siRNA designed to inhibit the mutated Transforming Growth Factor Beta I (TGFBI) to prevent the overproduction and aggregation of TGFBI protein in patients with Type II Corneal Dystrophy.
Corneal Dystrophies are a group of genetic, often progressive, eye disorders in which abnormal material accumulates in the clear (transparent) outer layer of the eye (cornea). Corneal dystrophy can cause watery eyes, dry eyes, sensitivity to light, pain, corneal erosion, and significant visual impairment, but to date there is no cure and treatment focuses on alleviating symptoms.
SIS-201-CD represents a first-in-class treatment to target the mutated TGFBI gene and inhibit protein accumulation in the cornea with the ease and convenience of eye drop administration.
SIS-202-CDC is a topical Bio-Courier CRISPR-Cas 9 designed to permanently edit the mutated Transforming Growth Factor Beta I (TGFBI) gene using double-cut CRISPR Cas 9 and stop the overproduction and aggregation of TGFBI protein in patients with type II Corneal Dystrophy.